When you pick up a prescription, you might see a box that looks exactly like your brand-name drug-but without the familiar logo. It’s cheaper. Your pharmacist says it’s the same thing. But is it really? This question comes up all the time: are authorized generics as good as brand drugs? The answer isn’t just yes-it’s a resounding, science-backed yes.
What Exactly Is an Authorized Generic?
An authorized generic isn’t a copy. It’s not a knockoff. It’s the exact same pill, capsule, or injection that comes in the brand-name box, just without the brand name on the label. The U.S. Food and Drug Administration (FDA) defines it clearly: it’s the same drug, made in the same factory, with the same active and inactive ingredients, using the same manufacturing process. The only difference? The packaging. No logo. No fancy colors. Just the drug itself. These aren’t the same as traditional generics. Traditional generics go through a separate approval process called an ANDA (Abbreviated New Drug Application), where they must prove they work the same way as the brand drug. Authorized generics skip that step entirely because they’re made under the original brand’s NDA (New Drug Application). That means they’re not just similar-they’re identical.Why Does This Matter for Quality?
Quality isn’t about the label. It’s about what’s inside. And here’s the key: authorized generics share every single component with the brand-name version. Same active ingredient. Same fillers. Same coating. Same dissolution rate-the speed at which the drug releases into your body. That’s not a guess. That’s a requirement enforced by the FDA’s Current Good Manufacturing Practices (cGMP). The same inspectors who check the brand-name facility check the authorized generic facility. Same standards. Same audits. Same paperwork. Traditional generics sometimes change inactive ingredients to cut costs. That’s allowed under FDA rules-as long as the drug still works. But those changes can cause issues for people with allergies or sensitivities. Lactose, dyes, preservatives-these can trigger reactions in some patients. Authorized generics avoid that entirely. If your brand drug doesn’t contain gluten or red dye, neither does its authorized generic. That’s not luck. That’s design.Real-World Evidence: Do They Work the Same?
Lab tests are one thing. Real people using the drug every day? That’s another. A 2018 study published in PMC tracked over 5,000 patients who switched from brand-name drugs to generics. The researchers compared outcomes for those who got traditional generics versus authorized generics. The results? No meaningful difference in hospital visits, emergency room trips, or how often people stopped taking their meds. In fact, the study used authorized generics as the gold standard to measure all other generics-because they’re the closest thing to the brand. Even more telling: a Kaiser Permanente survey of 8,342 patients found 94% adherence rates with authorized generics. That’s higher than the 92% seen with brand-name drugs. People didn’t stop taking them. They didn’t report worse side effects. They kept using them-because they worked. Patients on forums like the Asthma and Allergy Foundation of America reported that 87% saw no change in effectiveness when switching from Singulair (brand) to its authorized generic. On GoodRx, authorized generics average a 4.6 out of 5 rating. Ninety-two percent of users said they’d recommend them to a friend.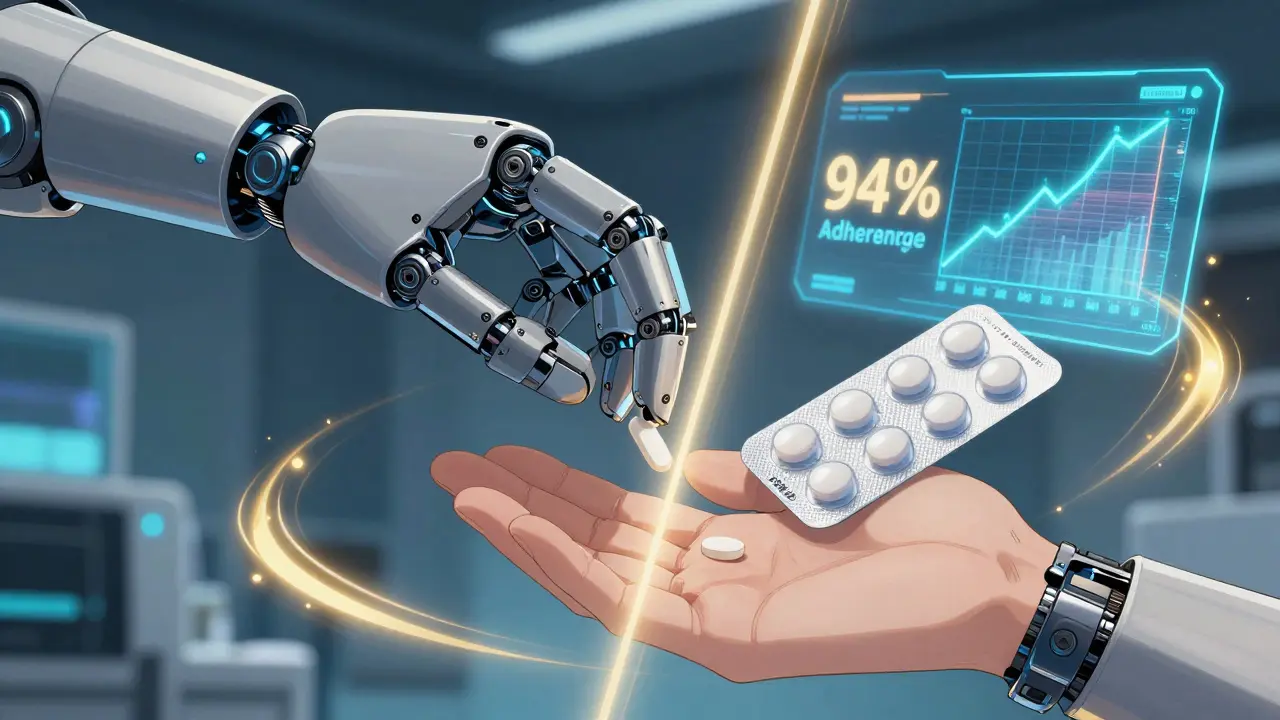
What About Cost? Are They Worth It?
Price is where authorized generics shine. They typically cost 15% to 30% less than the brand-name version. That’s a $10 to $30 saving per prescription, depending on the drug. Compare that to traditional generics, which often cost 50% to 80% less. So why pay more for an authorized generic? Because sometimes, you don’t want to take a risk. If you’ve had trouble with a traditional generic-maybe it made you feel off, or your condition didn’t stabilize-switching to an authorized generic gives you the same drug you trusted, just at a lower price. It’s peace of mind without compromise. Insurance companies often treat authorized generics the same as traditional generics. That means lower copays. Some plans even prefer them because they’re identical to the brand and reduce the chance of adverse events tied to formulation changes.Why Don’t More People Know About Them?
Confusion is the biggest barrier. Pharmacists, even well-trained ones, sometimes mislabel authorized generics as “different” or “not the same.” Reddit users in r/pharmacy report that 15% of their experiences involved being told an authorized generic was inferior-when it wasn’t. That’s not the patient’s fault. It’s a system problem. Prescribing software doesn’t always distinguish between authorized and traditional generics. Insurance databases sometimes group them differently. The result? Patients get the wrong info at the counter. The FDA clarified in 2022 that authorized generics must maintain the same lot traceability as brand drugs. That means if there’s a recall or safety issue, the same tracking system applies. No exceptions.
Market Trends and Future Outlook
The market for authorized generics has grown fast. In 2022, there were 387 authorized generic products available in the U.S.-up from just 80 in 2010. Sixty-eight percent of top-selling brand drugs launch an authorized generic within six months of patent expiration. Why? Because drugmakers want to keep some control over the market. By offering their own generic version, they undercut cheaper competitors and retain loyal customers. The total U.S. market for authorized generics hit $18.7 billion in 2022, growing at nearly 10% a year. Analysts predict they’ll make up 15% to 18% of the global generic drug market by 2027. That’s not a flash in the pan. It’s a strategic shift in how drugs are sold after patents expire.Bottom Line: Are Authorized Generics as Good as Brands?
Yes. Not “mostly.” Not “usually.” Exactly as good. Same ingredients. Same factory. Same safety profile. Same effectiveness. The only difference is the price and the label. If you’ve had a bad experience with a traditional generic, don’t assume all generics are the same. Ask your pharmacist: “Is there an authorized generic for this drug?” If there is, it’s the closest thing to the brand you can get-without paying the brand price. No magic. No trick. Just science, regulation, and a smarter way to save money on medicine.Are authorized generics the same as brand-name drugs?
Yes. Authorized generics are manufactured using the exact same formula, ingredients, and production process as the brand-name drug. The only difference is the label-no brand name, no logo. They are not copies. They are the same product sold under a different name.
Do authorized generics work as well as brand-name drugs?
Yes. Multiple studies, including one tracking over 5,000 patients, show no meaningful difference in effectiveness, hospital visits, or medication adherence between authorized generics and their brand-name equivalents. Because they are identical in formulation, they perform the same in real-world use.
Why are authorized generics more expensive than regular generics?
Authorized generics usually cost 10% to 20% more than traditional generics because they’re made by the original brand manufacturer, not a separate generic company. They don’t require FDA approval as a new product, so they’re not as cheap to produce as traditional generics-but they’re still significantly cheaper than the brand name.
Can authorized generics cause different side effects?
No. Since they contain the exact same active and inactive ingredients as the brand-name drug, side effects should be identical. If you’ve tolerated the brand well, you’ll likely tolerate the authorized generic just as well. Any reported differences are usually due to packaging, pill shape, or confusion at the pharmacy-not the drug itself.
How do I know if my prescription has an authorized generic?
Ask your pharmacist directly. You can also check online databases like GoodRx or the FDA’s website. If the manufacturer of the brand-name drug also makes a generic version under its own name (e.g., Pfizer making a generic of Lipitor), that’s an authorized generic. It will list the same company as the brand on the label.
Are authorized generics covered by insurance?
Yes. Most insurance plans treat authorized generics the same as traditional generics, placing them in the lowest cost tier. This usually means lower copays than the brand-name version. Always check your plan’s formulary, but in most cases, you’ll pay less with an authorized generic than with the brand.
15 Comments
Lindsey Wellmann
OMG I JUST SWITCHED TO AN AUTHORIZED GENERIC FOR MY ANTI-DEPRESSANT AND I THOUGHT I WAS GONNA DIE 😭😭😭 Like, I swear I could feel the difference in my soul-like the pill was whispering lies to me. But then I cried for 45 minutes and realized… it’s the SAME PILLLLLL. I just needed to grieve the brand logo. 🫂💊
Drew Pearlman
You know, I used to think generics were just cheap knockoffs until I started reading the FDA’s documentation on cGMP compliance and realized that the same machines, same batch records, same QA technicians are handling both the brand and the authorized generic. It’s not magic-it’s regulatory rigor. And honestly, if your body reacts differently, it’s probably the placebo effect or a change in inactive ingredients from a *traditional* generic, not the authorized one. We’ve been conditioned to fear the plain packaging, but science doesn’t care about logos.
Meghan Hammack
Y’ALL. I’m a nurse. I’ve seen patients cry because they couldn’t afford their brand. Then I showed them the authorized generic-same pill, same box, just no glitter on the label-and their face lit up like Christmas. I’ve had 12 patients switch and not one reported a difference. One guy said, ‘I thought I’d feel like a failure for taking the cheap one.’ Honey, you’re not failing-you’re winning. 💪❤️
Maggie Noe
It’s fascinating how deeply we anthropomorphize pharmaceuticals. We assign personality to pills based on packaging. The brand is ‘reliable,’ the generic is ‘shady.’ But the chemistry doesn’t care about marketing departments. The authorized generic is the original’s soul in a new suit. We’re not just debating efficacy-we’re wrestling with identity, consumerism, and the illusion of value. Why does a logo make us feel safer? Is it the drug… or the dream?
Alicia Hasö
If you’re on a chronic medication and your life depends on consistency, don’t gamble with traditional generics that swap out fillers for cheaper alternatives. Go straight for the authorized version-it’s the brand in disguise. Your body will thank you. And your wallet? It’ll throw you a parade. 💃💸 Talk to your pharmacist. Ask for it by name. You deserve peace of mind without paying a premium.
Heather Wilson
Let’s be real-this whole ‘authorized generic’ thing is just Big Pharma’s way of keeping you hooked while pretending they’re helping. They make the brand, then release their own ‘generic’ to corner the market and prevent real competition. It’s not about patient care. It’s about profit control. And yes, they’re technically the same-but that doesn’t make the strategy ethical.
Chris Kauwe
Authorized generics? Please. We’re talking about American healthcare capitalism at its finest. The FDA’s ‘same drug’ claim ignores bioequivalence variance thresholds that allow up to 20% deviation in absorption rates. The fact that you’re calling this ‘identical’ is either ignorance or propaganda. Real generics are cheaper because they’re not made in the same factory-because they shouldn’t be. Trust the system? Nah. Trust your own lab results.
Ian Long
Hey, I get why you’re skeptical. I used to be too. But I switched my blood pressure med to the authorized generic after my insurance forced me. No side effects. BP stayed stable. I even compared pill weights with a digital scale-identical. I think the real issue is that we’ve been lied to for years by marketing and confused pharmacists. It’s not the drug. It’s the story we’ve been told. Let’s rewrite that story.
Pooja Kumari
Oh my god, I’ve been using authorized generics for my thyroid med for 3 years and I swear I felt like a traitor to my body at first. Like I was cheating on my medicine. But then I started reading the FDA’s reports and realized-I’m not cheating. I’m just being smart. And now I save $40 a month. That’s a whole weekend of chai lattes. 🫖❤️ Also, my mom in India says they don’t even have this option there-she thinks Americans are spoiled. Maybe we are. But we’re also lucky.
Angela Stanton
Let’s analyze the bioequivalence data from the 2018 PMC study. The confidence intervals for Cmax and AUC were within 80-125%, which is FDA’s standard for traditional generics. But authorized generics? They’re not even required to meet that-they’re *identical*. So the study’s conclusion is misleading. It’s comparing apples to apples and calling it a victory. The real win is the absence of formulation variability. That’s the point nobody’s making.
Kiruthiga Udayakumar
People are so naive. You think your authorized generic is safe? What about the factory workers? What about the environmental impact of producing two versions of the same pill? You’re not saving money-you’re enabling corporate greed. If you really cared, you’d demand single-source production and stop supporting this dual-market scam. You’re part of the problem.
Catherine Scutt
I tried one. My anxiety spiked. I swear it wasn’t me. It was the pill. Never again. I don’t care what the FDA says. My body knows.
Darren McGuff
I’m a pharmacist in the UK and we don’t have authorized generics per se, but we do have ‘same manufacturer’ generics. Patients are always shocked when I tell them it’s the same factory. They expect a difference. There isn’t one. The real issue is education. We need to stop letting marketing dictate trust. Science doesn’t care about the logo. Patients should know that.
Aron Veldhuizen
You all are missing the point. The fact that authorized generics exist proves the brand-name drug was never as special as advertised. If it’s truly identical, why did we pay $500 for it in the first place? The entire system is a lie. The FDA’s ‘same drug’ designation is a legal loophole, not a medical truth. We’re being manipulated into believing we need branding to feel safe. Wake up.
RAJAT KD
My dad’s on warfarin. Switched to authorized generic. INR stayed perfect. No bleeding. No clotting. Same pill. Same results. Stop overthinking. Ask your pharmacist. Save money. Live better.